Chemical synthesis can be fun! No, really. Doing solid state chemistry in the lab can be an adventure, and sometimes you can even make some compounds that you didn’t know existed! Such is the story of my attempt (with cohort Dr. Dawnika Blatter of the USGS) to synthesize cobaltous oxide, a compound that I was hoping to use in future experiments. The journey involved heating and moulding silica glass tubes with a pair of exceptionally groovy welding goggles and an oxyhydrogen torch, a 1010 °C furnace, and some pretty funky colors.

Me and these all too fabulous welding goggles. Peace, love, and flame.
“Doesn’t Kayla study volcanoes?” you might be asking yourself. Yes, I do, and the cobaltous oxide is only tangentially related to my current study of Paektu volcano. The majority of my time is spent cooking up bits of volcanic rocks from Paektu in heated pressure vessels to map out how the mineralogy of the rocks differ depending on the pressure and temperature at which the rocks themselves formed. Basically, I create miniature magma chambers in the lab and try to determine which of my magma chambers best approximates the real one, which is currently sitting beneath the surface of North Korea and China and from which my rocks were ejected onto the surface some 1,000 years ago.
Long story short, the cobaltous oxide was to be used as a chemical buffer so that I could fix certain aspects of the environment in which I cook my rocks. In other words, I would use the CoO to control the chemistry of my synthetic magma chambers.
A Recipe for Co(II)O
In the lab, we had on hand powdered cobalt metal (Co), Co3O4, and various tools needed to perform solid state chemical synthesis. We modeled our plan after the way in which rhenium oxide has been successfully synthesized in this lab several times prior.
Cobalt comes in many oxidation states. It’s much like iron, which can exist as Fe metal, FeO, Fe2O3, or Fe3O4. Likewise, cobalt takes the forms Co metal, CoO, Co2O3, and Co3O4. At around at least 950 °C, the stable form of cobalt and oxygen is CoO (one mole of Co2+ bonded to one mole of oxygen makes one mole of CoO). At this temperature, our Co3O4 would break down to CoO and O2 in the following reaction:
2Co3O4 → 6CoO + O2
So, our recipe for making CoO was:
- Create a mixture of Co metal and Co3O4 with just enough Co metal to bond with all of the O2 liberated from the Co3O4 during the reaction above
- Enclose the mixture in an evacuated silica glass tube (evacuated meaning we removed all of the air from the tube, leaving only the mixture and empty vacuum)
- Heat the sealed, evacuated silica glass tube with mixture in a furnace above 950 °C for several days (hopefully enough time to facilitate the reaction)
- ???
- Profit!
Preparing the Silica Glass Tube
Working with the silica glass tube under an oxyhydrogen torch was probably the most fun part of this whole endeavor. It was also quite a light show.

Moulding the silica glass tube with an oxyhydrogen torch was the most fun part of this process
First, the silica glass tube would need to be sealed at one end. This was achieved by heating one end of the tube using an oxyhydrogen torch (O2 and H2 gas. That’s on fire.), pressing it down onto a cold aluminum plate, and repeating until the edges of the tube, softened under the flame, came together and sealed shut. Because the temperatures involved in this process are so hot, the silica glass tube would become incandescent when under the flame. It would glow so brightly that you could seriously damage your eyes without the use of very dark welding goggles. One cool effect that I noticed during this process was the way that the silica tube acted like a fiber optic cable. Light from the glowing end of the tube was completely internally reflected through the walls of the tube. This resulted in the cold end of the tube emitting the same white (or, through my goggles, blue) light as the hot end.
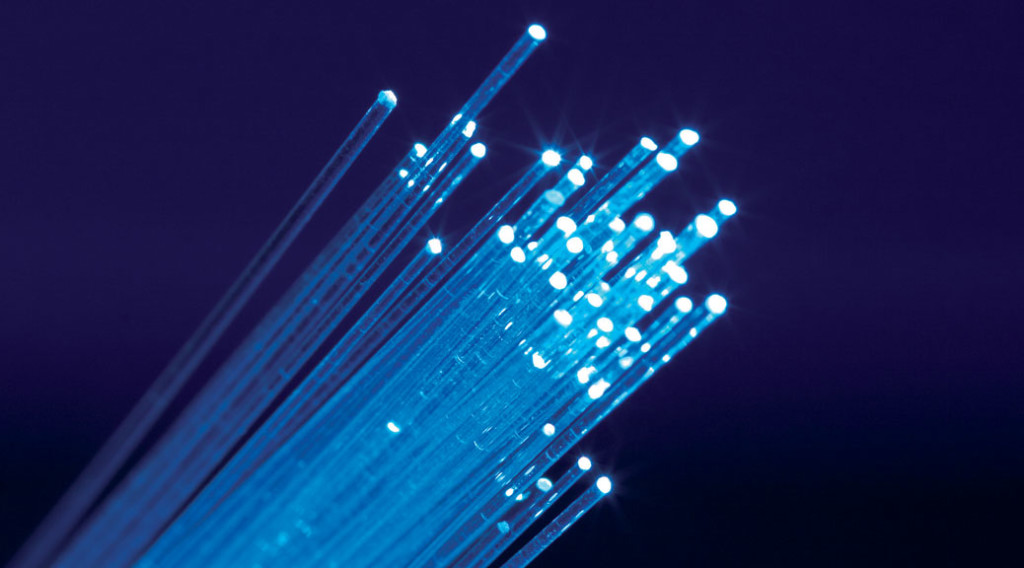
Fiber optic rods showing an effect like that seen during moulding of the silica glass tubes
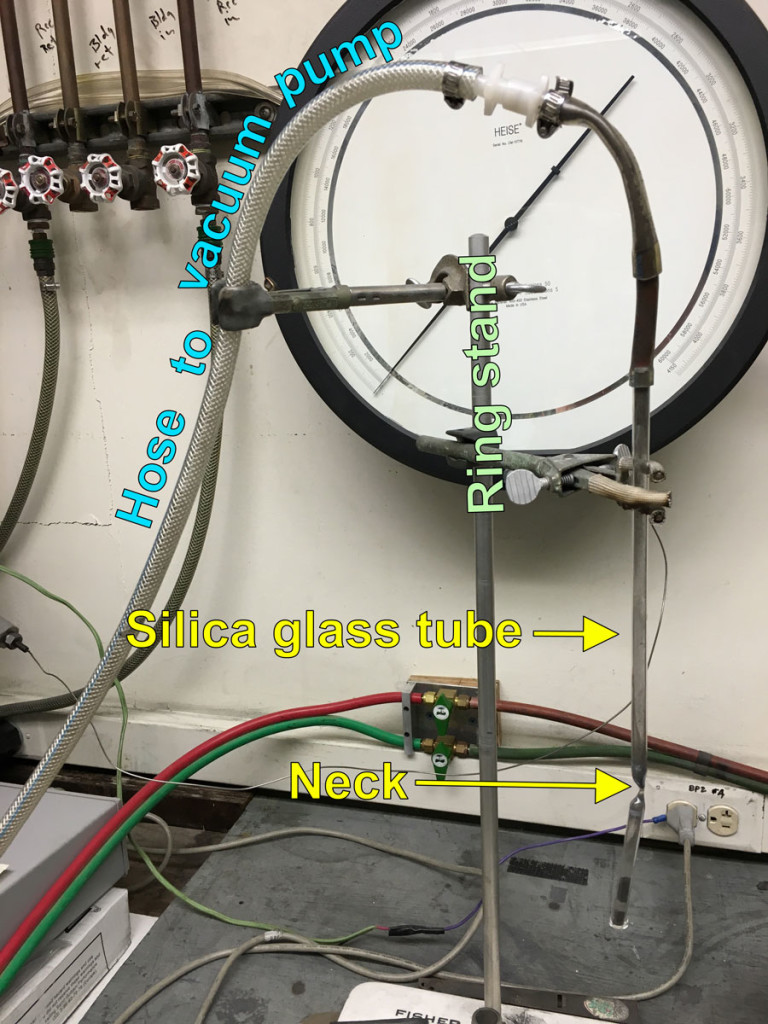
Next, our powder mixture was packed into the tube and the tube was evacuated. Evacuating the tube is a crucial step, as a tube full of air placed into a hot furnace would quickly undergo a large positive volume change and subsequently burst, sending glass shrapnel and cobalt powder everywhere. In order to remove the air from the tube and seal it, we would need a way to pull a vacuum on the tube without sucking all of the powder out in the process. To achieve this, we created a neck in the tube a few inches above the powder. The open end of the tube could then be attached to a vacuum pump via a hose, while the powder would theoretically remain at the bottom of the tube, the neck only allowing air to escape upwards and out of the tube. The necking part was nerve wracking and fun. Dawnika Blatter, partner in material synthesis, did the honors:
The next step was to pull a vacuum on the silica glass tube by attaching the open end to a roughing pump for about half an hour. Easy, right? Well, word to the wise, it is very easy to suck up the cobalt mixture powder unless the neck in your tube is very small. A T-junction attached to our setup probably would have helped, but we managed it in the end by v-e-r-y s-l-o-w-l-y attaching our rouging pump to our silica tube after several instances of a sort of powder fountain/explosion created when attaching the pump too swiftly. Here was our setup:
After about half an hour on the roughing pump, we sealed up the tube and shut off the pump. Sealing the tube was an interesting process that required holding the oxyhydrogen torch in one hand whilst simultaneously twisting and pulling on the bottom of the silica glass tube in the other. It was intense! Jessica Ball of Magma Cum Laude fame, joined us for this bit and took some photos:
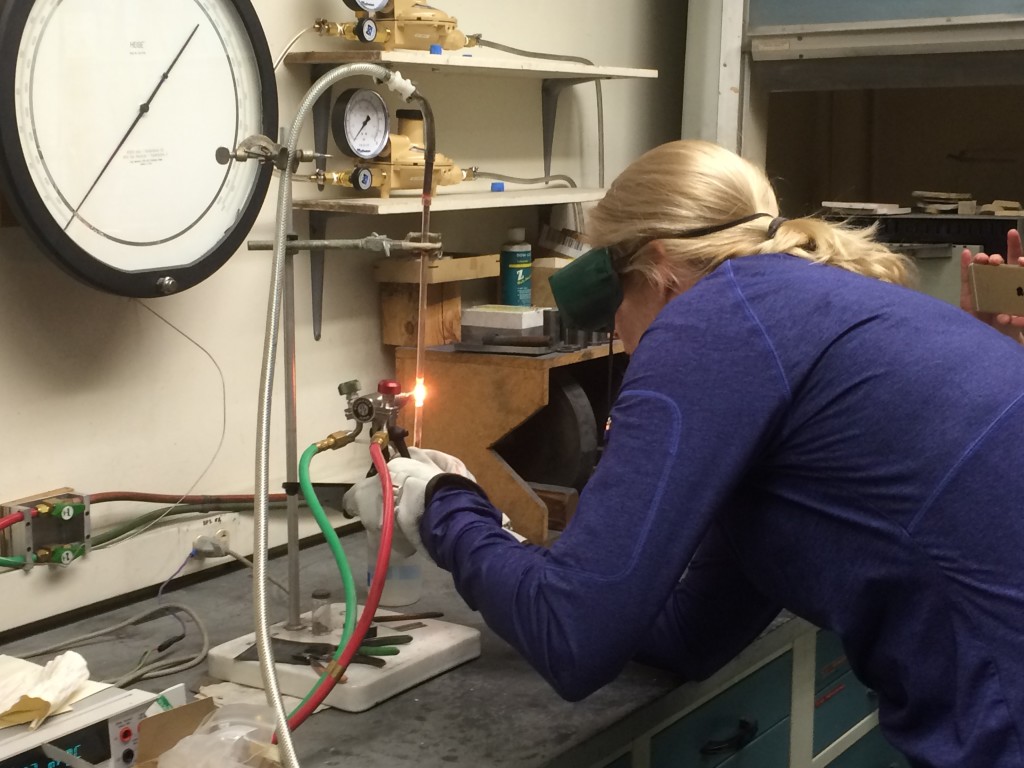
Dawkina sealing the silica glass tube. Photo by Jessica Ball
Meanwhile, I took video of the whole thing:
Success! Now we could cook our mixture in the furnace, leave it for a few days, and afterwards we should have CoO. And, that’s essentially what happened, with some additional astonishing results.
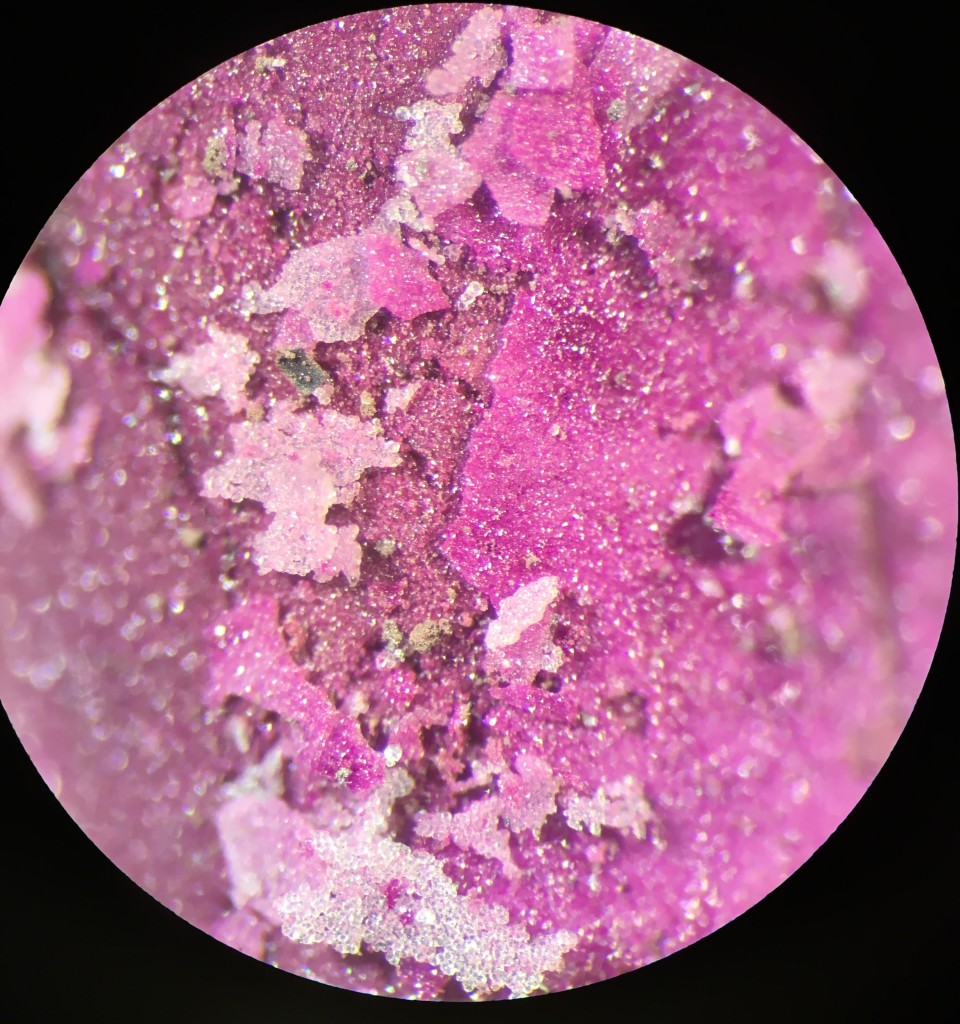
We made this…
What you’re looking at is a pellet of CoO (brown) encrusted with a rind of bright magenta cobalt orthosilicate crystals. Apparently, Co will bond with silica to make these crystals, members of the olivine family, which appear a really pretty purple-pink color. Of course, we made lots of silica available to the Co to drive this reaction (the silica glass tube!).
The second unexpected result of this experiment was the final form of the silica glass tube itself. When I removed it from the furnace, now at room temperature, I immediately noticed that the tube had ballooned to about twice the size it was before being put into the furnace. The best possible explanation I have come up with is that the O2 liberated during the breakdown reaction of Co3O4 around 950 °C caused a large enough volume change to pressurize the inside of the silica glass tube. The temperature, although about 1000 °C below the melting point of silica glass, must have been hot enough to soften the glass, allowing it to expand under pressure rather than burst. In the end, the tube grew from a diameter of 8 mm to about 12 mm, corresponding to a final volume 2.25 times that of the initial volume of the tube. I am really surprised that the glass would soften enough to allow for ductile (rather than brittle) behavior of the silica glass at such a low temperature (experimental temperature 1010 °C).

The silica glass tube after our synthesis experiment (top) compared to its diameter before the experiment (bottom)
Unfortunately, I am not satisfied with the product I made. Don’t get me wrong, it’s totally cool! Funky, even! But, the cobalt orthosilicate is very difficult to remove from the CoO, as the whole pellet is quite friable. And what little CoO I did recover has small metallic granules distributed throughout. My guess is that those granules are Co metal, indicating an incomplete reaction. In the end, I’ve decided it’s best to simply order the CoO from our chemical catalogue. It’s not the cheapest stuff on the market, but I guess it’s worth the price!


Comments are closed